
The formal charge is defined as the charge we assign to a given atom inside a molecule following the assumption that electrons are shared equally among elements for bond formation. Step 5: Before we can confirm a certain Lewis Structure diagram, we have to check one last thing and that is the Formal Charge. In the diagram, we can see that hydrogen has got two electrons surrounding its atom and therefore this criterion has also been fulfilled. In the case of hydrogen, however, we see that it will have a tendency to have two electrons in its outermost shell as it will achieve configuration.
As per the above sketch, we have fulfilled the required criterion. It says that the elements of group 1 to group 17 tend to achieve the octet outer shell electron configuration of noble gas elements.Īs we can see in HF, we have fluorine which will tend to have eight electrons in its valence shell. The octet rule deals with the octet fulfillment of valence shells of elements. Step 4: Now, we will check the octet rule. Let us have a look at the schematic sketch of HF after we have placed the dot electrons: Step 3: Lewis Structure is also known as an electron-dot structure since we use dot notations to represent the valence electrons surrounding the atoms. Hydrogen fluoride is a diatomic molecule, so here the concept of a central atom does not take place. Usually, the element having the least electronegativity value acts as the central atom. Step 2: Following the general procedure, now that we have found the valence electron number, we have to work out the element which will take the central position in the molecule. Total number of valence electrons in an HF molecule = 1 + 7 = 8. We can check the atomic number from the periodic table to confirm the valence electron number. Hydrogen belongs to period 1 and has 1 valence electron whereas Fluorine belongs to period 17(group of halogens) and therefore has 7 valence electrons. HF has one atom of hydrogen and one atom of fluorine. Step 1: We have to first count the total number of valence electrons inside a single hydrogen fluoride molecule. Let us discuss the step-by-step procedure to draw the Lewis Structure diagram for an HF molecule: the electrons in the outermost shell of the atom which take part in bonding. Here, we will talk about the valence electrons i.e. Lewis Structure gives a 2-dimensional sketch representing the distribution of electrons among atoms inside a molecule. To learn the nature of bonding inside a molecule of HF, we will first understand the concept of Lewis Structure so that we can draw the correct diagram. The science behind the formation of chemical bonds helps us understand several chemical and physical properties exhibited by different molecules and compounds. The below reaction provides us the procedure of formation of HF:ĬaF2 + H2SO4 -–> CaSO4 + 2HF (endothermic reaction)Ĭhemical bonding is the study of atomic attraction that results in the formation of new products. Hydrogen fluoride gas is dangerous and aqueous HF acid is corrosive and toxic. Also, hydrogen fluoride can be used to manufacture herbicides, fluorescent light bulbs, refrigerants, and so on. Other than this, it also acts as the precursor of several metal fluorides like aluminum fluoride and uranium hexafluoride.Īnhydrous HF has catalyzing properties and is hence used in the process of petroleum alkylation to increase the octane number of petroleum. It acts as a precursor to the halogen fluorine via electrolysis procedure. HF has a molar mass of 20.0064 g/mol and a density of 1.15 g/litre as a gas at 250 C. It has a melting point of -118.50 F and a boiling point of about 670 F.

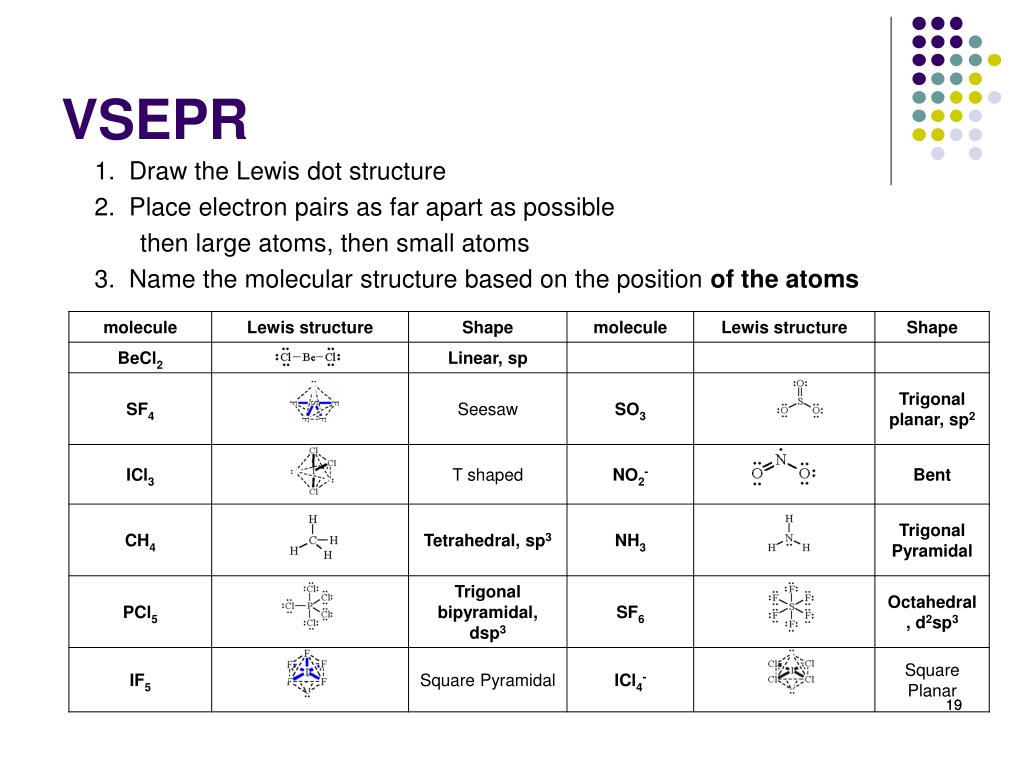
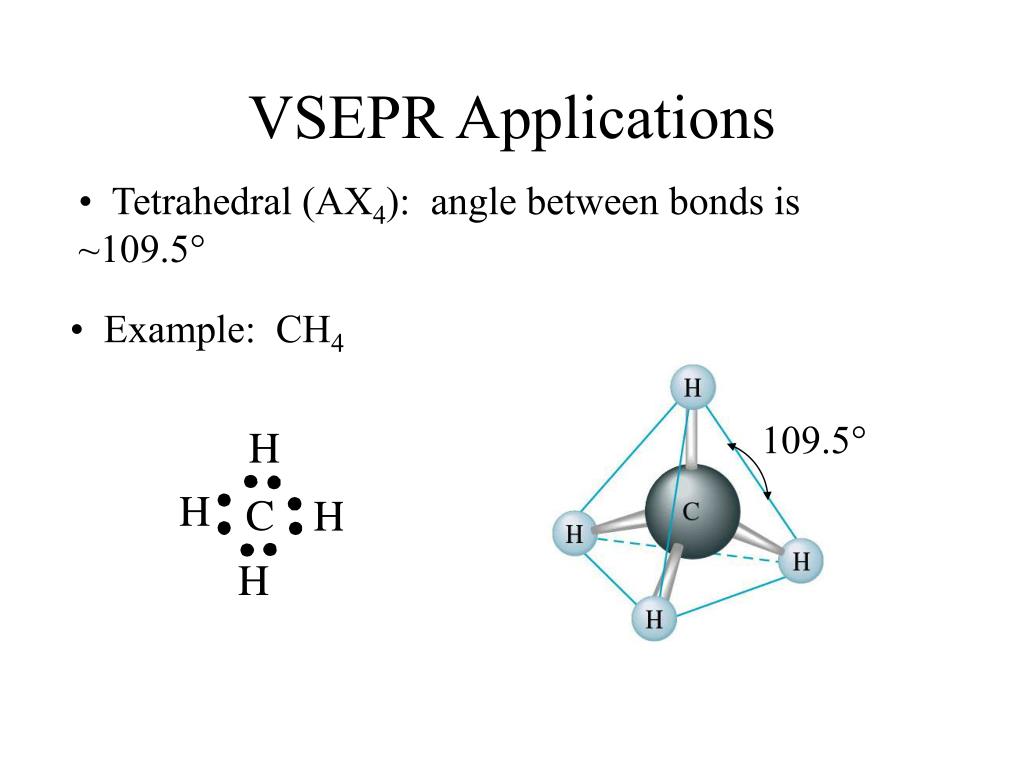
It tends to dissolve in water and the colorless aqueous solution is known as hydrofluoric acid. However, if a VSEPR structure is generated from a provided image, in which an organic molecule is symbolized using an alternative notation, the relative placement of each atom must remain consistent between both representations.Hydrogen fluoride is a colorless liquid or a gaseous compound having the chemical formula HF. When connecting atoms using VSEPR notation, the second carbon can be bonded to any "side" of the first elemental symbol that is written, but the connectivity of all subsequent carbon atoms must be considered relative to those that are already present. In order to represent the bond that exists between consecutive atoms, a line that connects their corresponding elemental symbols should be drawn. \): Two three-dimensional VSEPR representations of a tetrahedral carbon atom.īecause all organic molecules must contain carbon, C, this element should be written first when symbolizing an organic substance using VSEPR notation.


 0 kommentar(er)
0 kommentar(er)
